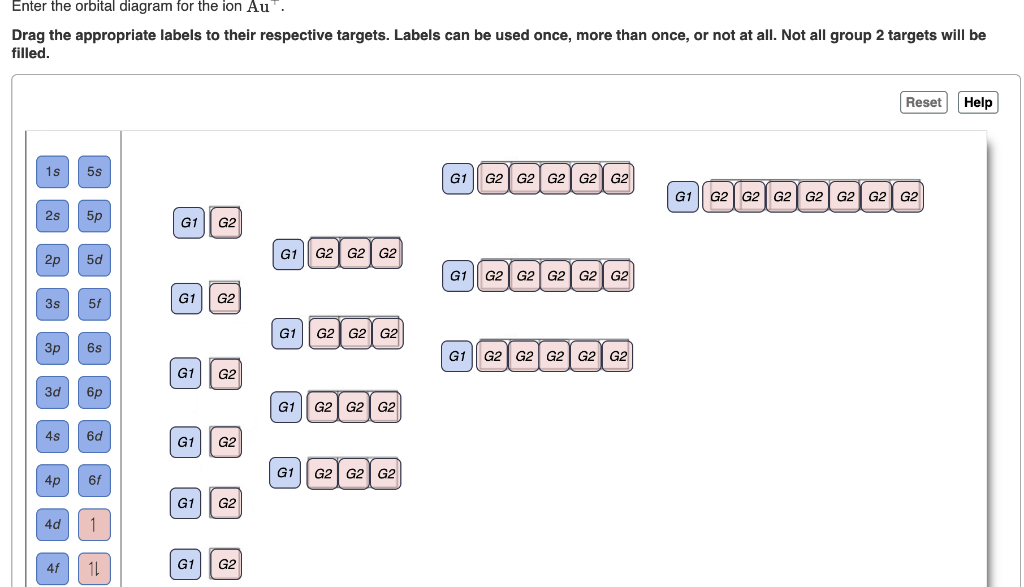
Enter the Orbital Diagram for the Ion Au+
The number of unpaired electrons for any free atoms or ions can be determined by using the orbital diagram. In each box can be two arrows with opposite spin maximum.
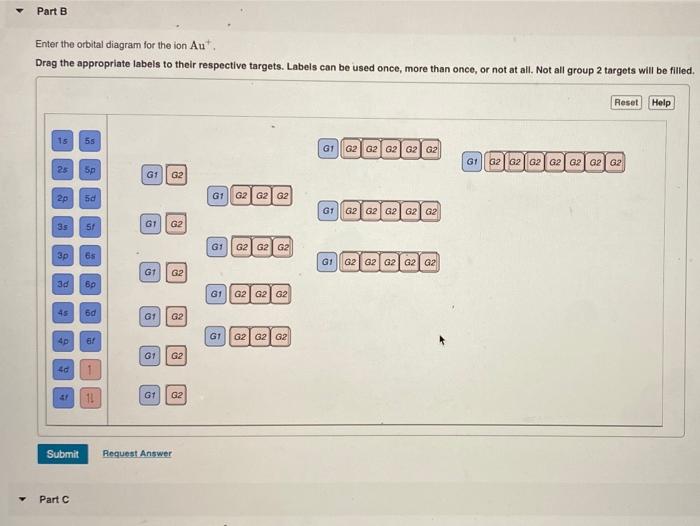
Solved Part B Enter The Orbital Diagram For The Ion Au Drag Chegg Com
LIMITED TIME OFFER.
. Add them in order of increasing orbital energy. Orbital diagram for Au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1. Using a partial orbital diagram show.
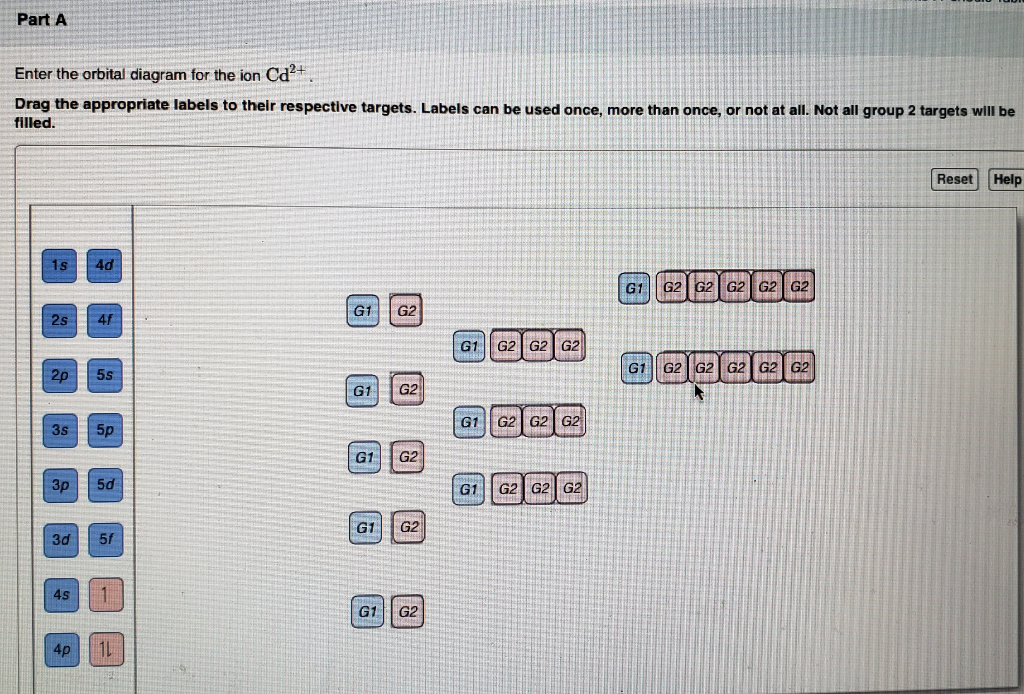
Enter the orbital diagram for the ion Cd2. Enter the orbital diagram for the ion Au. You just have to fill the boxes with arrows s orbital has only one box with two arrows.
GET 20 OFF GRADE YEARLY SUBSCRIPTION. Electrons are generally removed from the s sub-level 1 Remove one electron from 5s1 ANSWER. Drag the appropriate labels to their respective targets.
Enter the orbital diagram for the ion AuAu. Use the buttons at the top of the tool to add orbitals. Electron Configuration Electron Configuration Watch on Electron Configuration Xe 4f14 5d10 6s1.
An orbital diagram is the pictorial representation of shells in an atom by using square boxes one box for s-orbital three boxes for p-orbitals five boxes for d-orbitals and seven boxes for f-orbitals those boxes are filled by electrons using the following. Exampleparamagnetic diamagnetic etc. Use the buttons at the top of the tool to add orbitals.
Add them in order of increasingwrite orbital diagram for each ion and determine if the ion is diamagnetic or paramagnetic. Labels can be used once more than once or not at. Zr 2 Provide your answer.
Enter the orbital diagram for the ion au. Therefore its configuration is. Orbital diagram for Au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1 you just have to fill the boxes with arrows s orbitalOrbital diagrams of atoms Diagram shows how the electrons are distributed among.
Enter the orbital diagram for the ion Au When an element is a cation you REMOVE electrons. Up to 256 cash back Get the detailed answer. Answer to Write orbital diagram for Mo3.
An orbital diagram is the pictorial representation of shells in an atom by using square boxes one box for s-orbital three boxes for p-orbitals five boxes for d-orbitals and seven boxes for f-orbitals those boxes are filled by electrons using the following principles. Click within the orbital to add electrons. For Au one electron is removed from the outermost 6s orbital making the configuration Xe4f 145d10.
Answer to Write orbital diagram for Au. P has 3orbitals with 6arrows. Draw an Molecular Orbital energy diagram and predict the bond order of L 2.
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 Enter the orbital diagram for the ion Mo3 When an element is a cation you REMOVE electrons. The atomic number of Au is 79. D has 5 boxes with 10 arrows and f has 7boxes with 14 arrows.
The atomic number of Au is Therefore its For Au one electron is removed from the outermost 6s orbital making the configuration. The number of unpaired electrons for any free atoms or ions can be determined by using the orbital diagram. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1.
Enter the orbital diagram for the ion au.
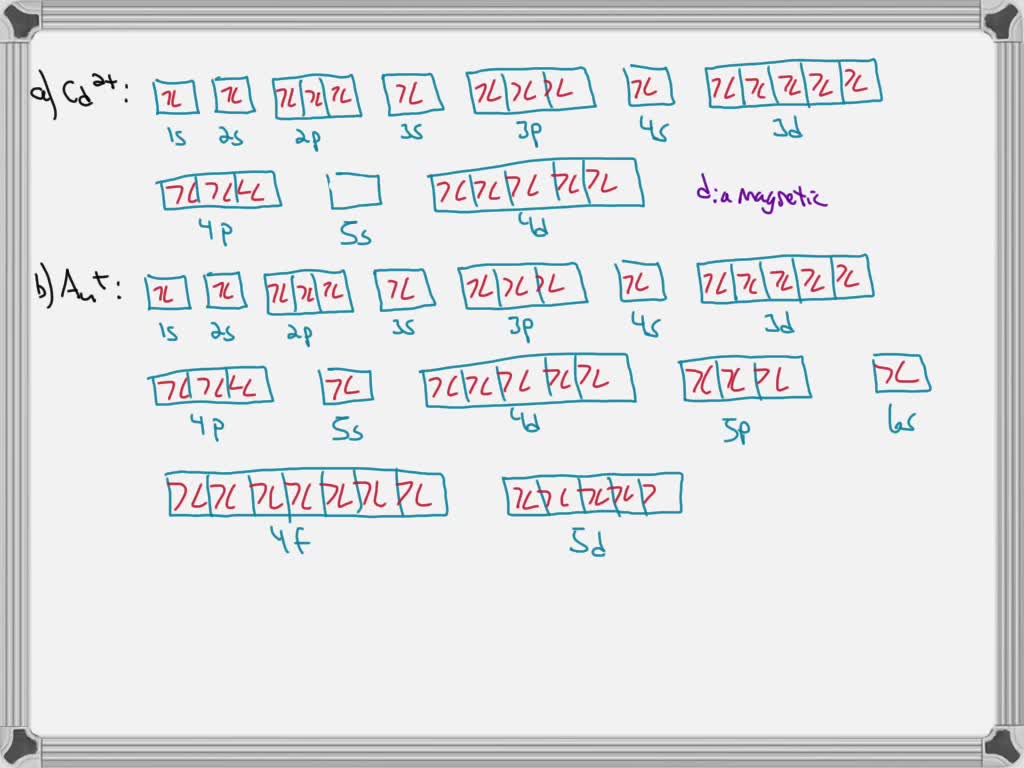
Solved Write Orbital Diagrams For Each Ion And Determine If The Ion Is Diamagnetic Or Paramagnetic A Cd2 B Au C Mo3 D Zr2
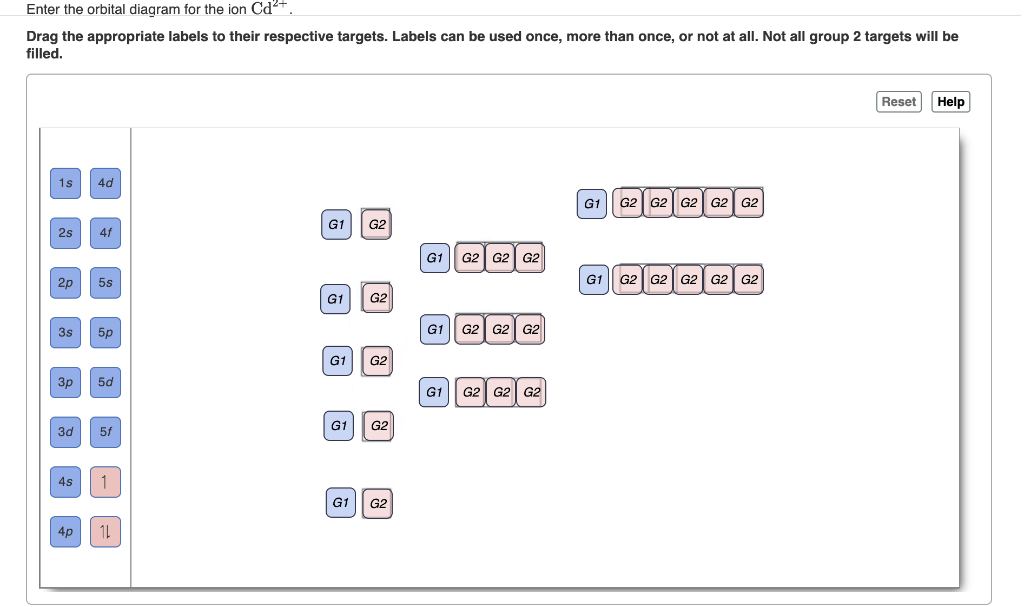
Solved Enter The Orbital Diagram For The Ion Cd2 Drag The Chegg Com
Solved Enter The Orbital Diagram For The Ion Cd2 Drag The Chegg Com

Solved 1 Enter The Orbital Diagram For The Ion Cd2 Drag Chegg Com

Comments
Post a Comment